Introduction: A polypill containing all four classes of guideline-directed medical therapy (GDMT) for heart failure with reduced ejection (HFrEF) has been proposed to change the heart failure treatment paradigm. The acceptability, appropriateness, and feasibility of a HFrEF polypill-based strategy is unknown. The purpose of this study was to elicit patients’ and providers’ priorities in the design of HFrEF polypills in the U.S.
Methods: From April 2023 to December 2023, we conducted a convergent mixed-methods study at Washington University in St. Louis (WUSTL), University of California, San Francisco (UCSF), and the American College of Cardiology (ACC). We administered physician surveys using adapted implementation outcome measures and conducted in-depth interviews with patients, cardiologists, general internists, and advanced practice providers. We conducted thematic qualitative analysis guided by the Consolidated Framework for Implementation Research v2.0.
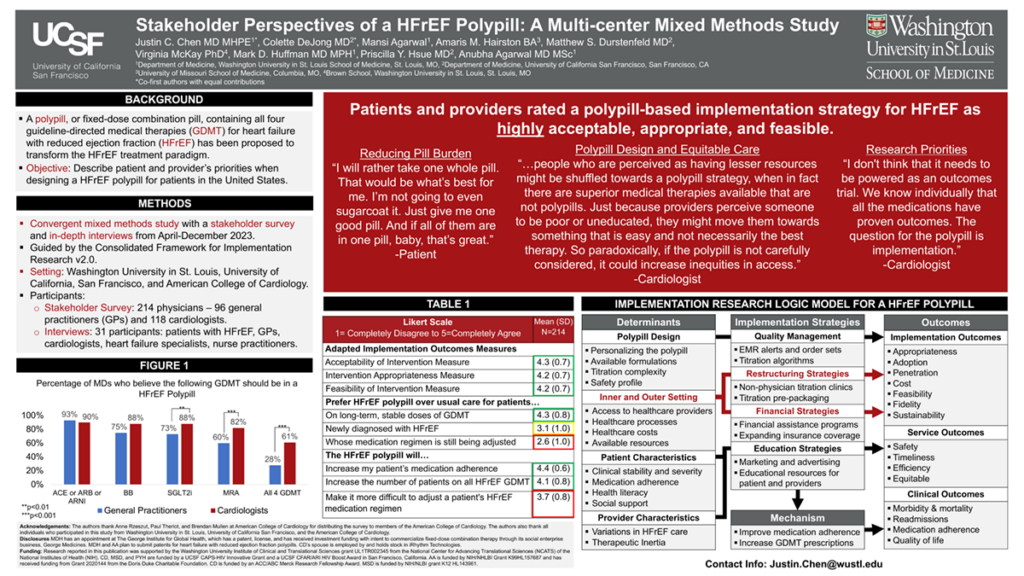
Results: Out of 224 survey respondents across the United States, participants agreed that HFrEF polypills are highly acceptable (mean [SD], 4.2 [0.7]), highly appropriate (4.1 [0.8]), and highly feasible (4.1 [0.7]). Key themes emerged from interviews with patients and providers including 1) medication adherence, variations in clinical practice, and access to healthcare are determinants of HFrEF care, 2) variations in HFrEF polypill design may influence uptake, 3) equitable implementation of HFrEF polypills will be influenced by cost, and 4) future research priorities including evaluation of effectiveness and implementation.
Impact: A HFrEF polypill-based strategy was acceptable, appropriate, and feasible by patients and physicians. Participants described key priorities, including HFrEF polypill components, titratability, and effects on health equity, that will inform the design and implementation of future trials.
Organization: Washington University in St. Louis
Chen JC, DeJong C, Agarwal M, Hairston AM, Durstenfeld MS, McKay V, Huffman MD, Hsue PY, Agarwal A