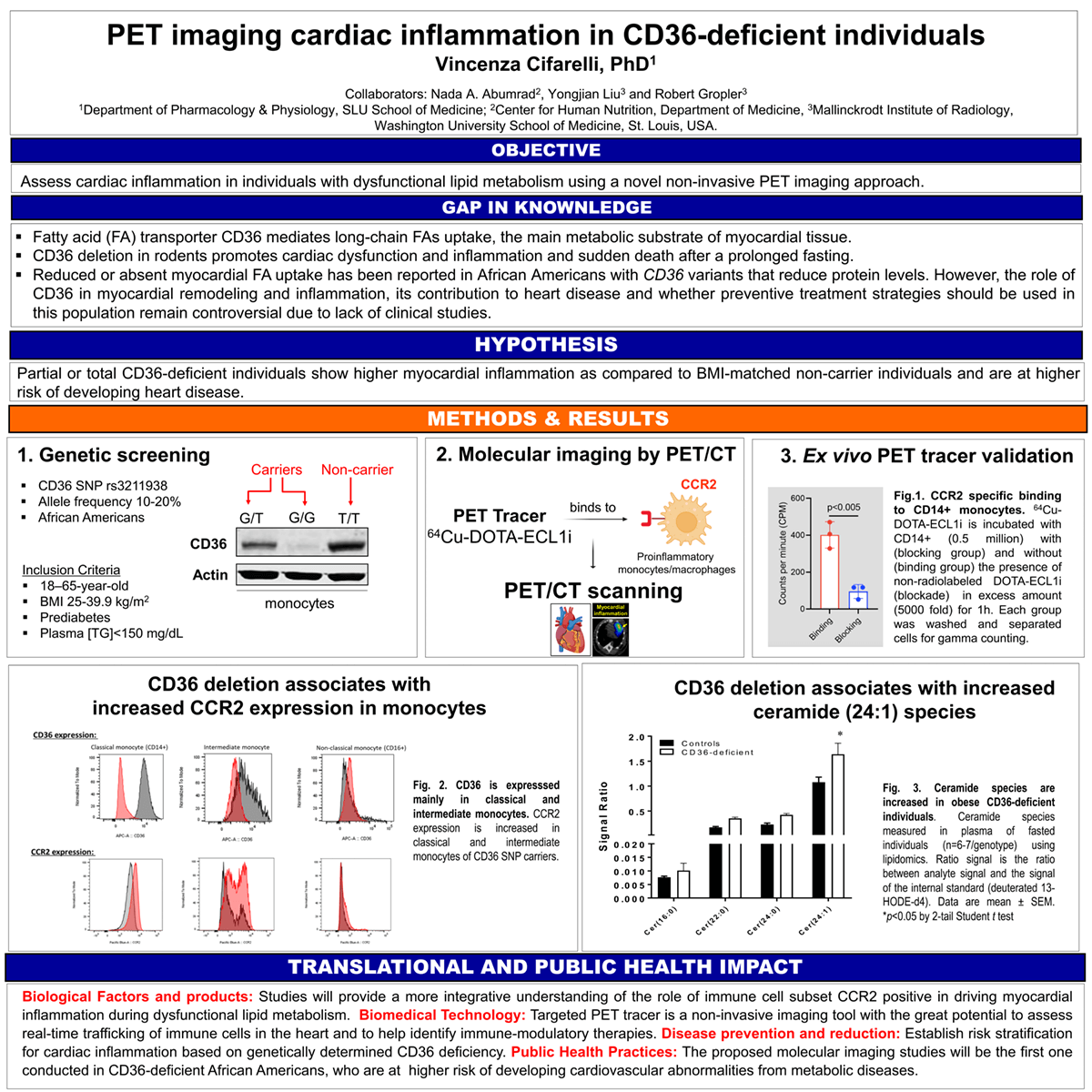
Introduction: Fatty acid translocase CD36 regulates cardiac function, and its deletion in rodents associates with atrioventricular block and bradycardia and increasing risk of sudden death following prolonged fasting. Preclinical and clinical research show that monocyte chemotactic protein-1/chemokine (C-C motif) receptor 2 (MCP-1/CCR2) regulates myocardial remodeling and inflammation during cardiac disease. Our preliminary data, conducted with a specific CCR2 binding PET radiotracer, 64Cu-DOTA-ECL1i, show higher monocyte/macrophage (CCR2+) infiltration in CD36 null hearts at steady state as compared to age- and sex-matched wild-type mice. In African Americans, CD36 genetic variants associate with dyslipidemia, vascular stiffness, and cardiovascular disease. However, the role of CD36 in myocardial remodeling and inflammation, its contribution to heart disease and whether preventive treatment strategies should be used in this population remain controversial due to lack of clinical studies.
Methods: Obese African American carriers and BMI-matched non-carriers (n=14) of the coding single nucleotide polymorphisms rs3211938, (minor allele frequency ~20%), will receive 64Cu-DOTA-ECL1i by infusion for PET/CT imaging. Results will be validated by 1) radiotracer binding profile ex vivo in isolated monocyte by autoradiography and 2) CCR2 expression in monocytes subsets by flow cytometry.
Results: As compared to non-carriers, CD36 carriers present 1) increase expression of CCR2 in classical and intermediate monocytes; 2) increased level of monocytes (CD14+), and 3) increased plasma ceramides species typical of coronary heart disease and heart failure.
Impact: This will be the first molecular imaging study assessing myocardial inflammation in obese individuals with CD36-deficiency and will provide a more integrative understanding of how immune cell subsets, in particularly CCR2+, drive myocardial inflammation during dysfunctional lipid metabolism. Results from these studies will serve as the foundation for future investigations determining how the myocardial inflammation drives cardiac pathology and identify potential therapeutic approaches to ameliorate the process.
Organization: Saint Louis University
Cifarelli V, Abumrad NA, Liu Y, Gropler R