Stroke Patient Access Center (SPAC)
Provides resources for stroke clinical research, including feasibility analysis, regulatory and recruitment support, randomization, enrollment, retention, and study closure.

Overview
- Our mission is to support the advancement of stroke and cerebrovascular clinical research, with the ultimate goal of improving the care of our patients affected by stroke and cerebrovascular disease.
- The Stroke Patient Access Core (SPAC) provides investigators with a centralized infrastructure for all aspects of stroke clinical research. These include research feasibility analysis, regulatory responsibilities, recruitment, consenting, randomization, enrollment, follow-up, retention, and study closure.
Goals
- Investigating the most effective and safe ways to save more brain and improve patient outcomes after suffering a stroke.
The Urgency Behind Strokes
- Research Feasibility Analysis: The SPAC team can provide analysis and evaluation through protocol review to determine the recruitment strategy for the individual study to optimize patient enrollment. The feasibility study will also provide information that may project study enrollment.
- Regulatory: Preparation of IRB submissions and interaction with the Human Research Studies Committee; and interaction with study sponsor.
- Recruitment: The screening process includes screening of daily acute ED stroke pages, all neurology inpatients, and stroke clinic outpatients. Most screens occur daily in real-time or at multiple time-points. We will approach all suitable candidates that meet criteria for the proposed study.
- Consent: The study consent may be presented to the patient, family, or significant others. The patient, family or significant others should understand the purpose, procedures, and the potential risks and benefits of the study through the consent process. The patient or the legal authorized representative may be consented. The consent process will be documented according to the federal guidelines (45 CFR 46.116).
- Randomization: After completion and documentation of the consent process, we will randomize the patient according to the specific protocol requirements.
- Enrollment: Patients may be enrolled per the specific protocol guidelines.
- Follow-up: Visits will be scheduled according to the individual protocol schedule of events provided in the protocol.
- Retention: Initial patient interview will include at least two alternate phone numbers for family or significant others. Phone calls may be made to retain the patient and confirm follow-up visits.
- Study Closure: Complete necessary patient final visits and end of study documents.
- Data recording and data entry: We collect and track information, create source documents and work with the sponsor to streamline data recording and data entry.
- Acute Intervention Trials:
- Acute interventional trials may include studies examining investigational drugs or devices used in conjunction with, or in place of, the standard of care practice, such as thrombolysis administration or mechanical thrombectomy.
- Recovery Trials:
- Stroke is the leading cause of disability in Americans, and recovery trials give stroke survivors the opportunity to improve significant physical, cognitive, and emotional deficits that may have impacted the patient while experiencing a stroke.
- Prevention Trials:
- Prevention trials are vital to help identify optimal treatment strategies that may reduce the risk of having a recurrent stroke.
- Additionally, prevention trials may target managing and controlling vascular risk factors (diabetes, smoking, high blood pressure, high cholesterol and lipid levels, etc.) that prove to largely impact the onset of future strokes.
- Observational Trials:
- Examining and measuring specific biomarkers, such as brain imaging and cognition, in patients with strokes or cerebrovascular diseases to determine health outcomes.
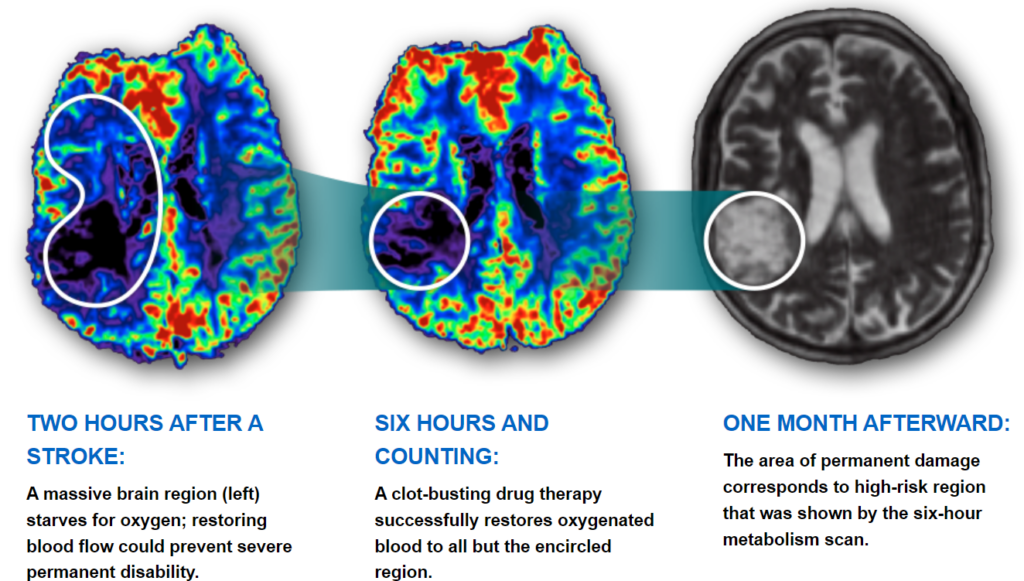
- Stroke Effects on the Brain Through Time:
- Mark VCID:
- Goal: discover potential biomarkers involved in small vessel diseases of the brain that impact cognitive impairment and the development of dementia. This will allow for a better understanding of and ways to treat these impairments in the future.
- StrokeNet:
- Goal: NIH StrokeNet is a network consisting of 27 regional centers across the United States and overseas relations that conducts small and large clinical research studies and trials to create advances in future stroke treatment, prevention, recovery, and rehabilitation.
- RVCL:
- Goal: to create awareness and improve patient care through clinical studies and drug trials for the newly discovered disease known as retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations (RVCL-S).
- WUSTL Stroke
- ECRC:
- Our core closely collaborates with Emergency Care Research Core (ECRC) by enrolling patients in hyperacute research trials directly in the emergency department.

Professor of Neurology, Professor of Radiology Department of Neurology
Division of Stroke and Cerebrovascular Disease
Institute of Clinical and Translational Sciences
Phone: 314-362-7382
Email: forda@wustl.edu

Clinical Research Specialist
Department of Neurology
Phone: 314-747-8794;
314-369-6880
Email: babkaj@wustl.edu

Research Nurse Coordinator
Department of Neurology
Phone: 314-299-8638
Email: lindsayt@wustl.edu

Department of Neurology
Phone: 314-273-3498
Email: angelaw@wustl.edu
To learn about SPAC services, please contact Jenny Babka, RN, BSN, at 314-747-8794 or babkaj@wustl.edu.
Location: McMillan Building, 5th floor, Washington University School of Medicine Campus