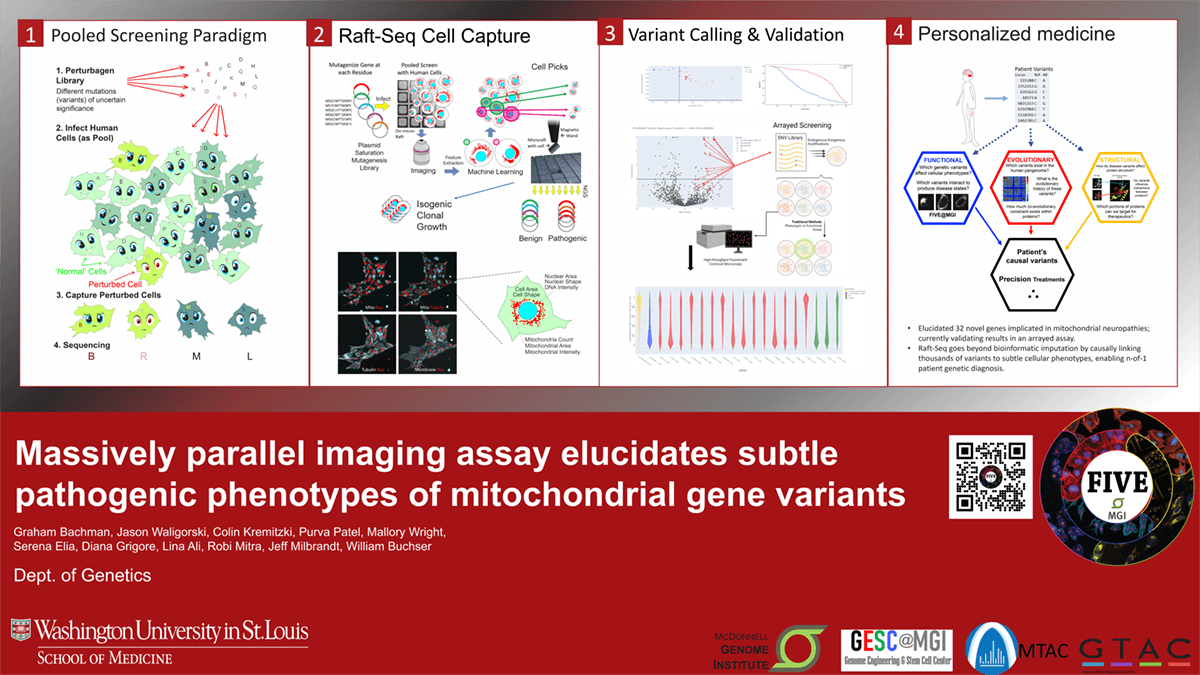
Introduction: Mutations in mitochondrial genes underlie numerous neurodegenerative diseases, yet the significance of most variants is uncertain concerning disease phenotypes. Many pathogenic mutations cause visibly distinct morphological differences in afflicted cells, allowing human cells to serve as proxies for patients when studying the genetic basis of disease. This postulate provides the basis for a pooled functional screening platform called “Raft-Seq” that maps cellular phenotypes to specific variants with high-throughput, enabling n-of-1 patient genetic diagnosis in precision medicine.
Methods: The Raft-Seq pipeline uses a library of gene knock-out gRNAs introduced to cells by CRISPR/Cas9. Cells are plated onto microraft arrays, stained, imaged, and traced to generate training data for machine learning classifiers. These models identify cells-of-interest, which are isolated, expanded, and sequenced. Cell sequence and feature data are compiled and analyzed to identify pathogenic variants, which are validated in an arrayed assay.
Results: We (FIVE@MGI, an ICTS core GESC@MGI affiliate) used Raft-Seq to target numerous mitochondrial genes and investigate their impact on cellular mitochondrial pathology in human cells. Of the 1,102 screened genes, 32 were identified as pathogenic variants and are currently being validated. We also endeavored to identify variants of interest within a library of 225 knock-out gRNAs targeting the ATAD3A and SLC25A19 gene, both of which are implicated in clinical neuropathologies related to mitochondria.
Impact: Identifying the cause of a patient’s idiopathic disease can end their diagnostic odyssey. Raft-Seq goes beyond bioinformatic imputation of possible pathogenicity by functionally screening patient variants to determine their impact on disease, causally linking thousands of gene edits to complex and subtle cellular phenotypes. We have used this platform to elucidate novel genes implicated in mitochondrial neuropathologies and to clarify the pathogenicity and phenotype of hundreds of ATAD3A and SLC25A19 variants. These studies set the stage for new genomic diagnostics and therapeutic discovery.
Organization: Washington University in St. Louis
Bachman GW, Kremitzki C, Waligorski J, Patel P, Bramley J, Vakaki M, Chandrasekaren V, Ali L, Elia S, Grigore D, Wright M, Mitra R, Milbrandt J, Buchser W