Introduction: An unplanned pregnancy can be an emotionally devastating experience for a woman. To expand non-hormonal choices for female-controlled contraceptive methods, this research aims to develop a drug-free, tampon-like xerogel device capable of fortifying natural contraceptive mechanisms within the female reproductive tract.
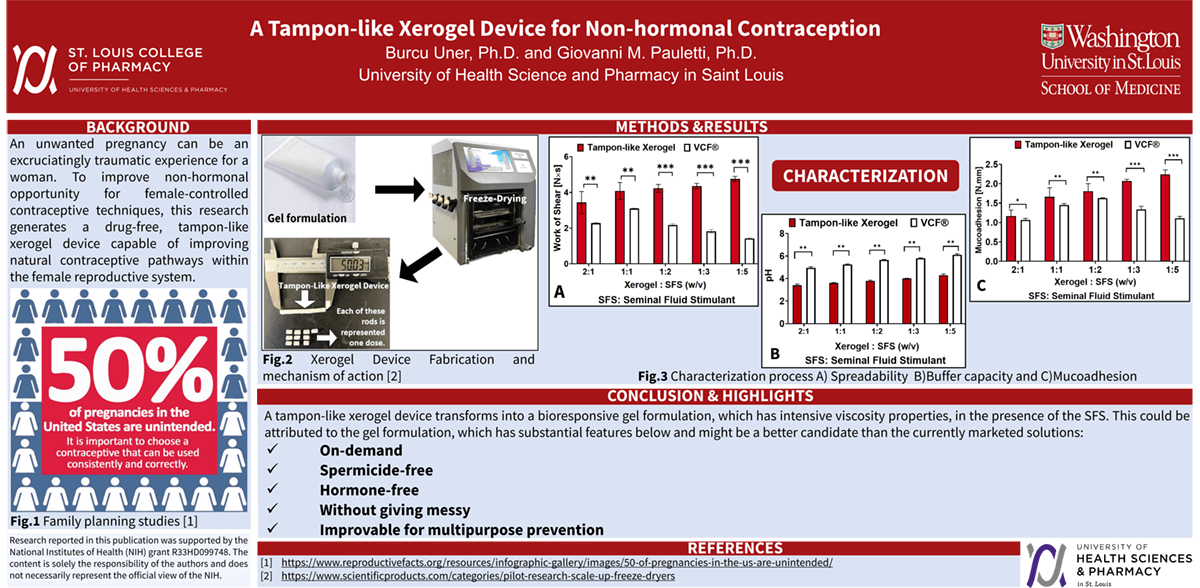
Methods: Xerogel devices were fabricated by lyophilization of a hydrogel comprised of Carbopol® 974P (4%, w/w), polyvinylpyrrolidone (4%, w/w), and D-mannitol (3%, w/w). Viscoelastic and mucoadhesive properties of xerogel devices following exposure to vaginal fluid simulant, pH 4.2, (VFS) and seminal fluid simulant, pH 7.7, (SFS) were quantified using the TA-XT Plus Texture Analyzer. In parallel, buffer capacity was measured using a DeltaTrak ISFET pH probe. Device porosity was estimated using volumetric displacement of n-hexane.
Results: The porosity of lyophilized xerogel devices was 72-77%. Exposure of xerogel devices to VFS rapidly established a partially hydrated gel phase, which exhibited mucoadhesive properties that were 2-fold greater than those measured for the marketed contraceptive VCF® gel. Viscoelastic properties of the VFS-hydrated xerogel device dramatically increased by 160% upon exposure to SFS. Under those conditions, however, the pH value of the gel phase only moderately increased from pH 3.4 to pH 4.8.
Impact: Vaginal administration of this tampon-like xerogel device fabricated by lyophilization rapidly establishes an acidic, bioresponsive gel phase that increases in viscoelastic properties upon exposure to alkaline seminal fluid. Combined with the pronounced buffer capacity of the acidic gel phase, this novel, drug-free bioengineering concept is predicted to exhibit significant contraceptive efficacy in vivo by fortifying the natural contraceptive mechanisms within the female reproductive tract.
Organization: University of Health Sciences and Pharmacy
Uner B, Pauletti GM