Returning genetic results to research participants requires thoughtful planning. ICTS Precision Health at Washington University in St. Louis, aims to catalyze genomic research by providing grant review and development services, guidance and resources for genomic researchers and genomics education in the community. Precision Health is a component of the Institute of Clinical and Translational Sciences’ Clinical and Translational Science Award, granted by the National Center for Advancing Translational Sciences at the NIH.
What is Precision Health and how does it work with the ICTS?

Precision health is a concept that takes into account differences in people’s genes, environments, and lifestyles and formulates treatment and prevention strategies based on patients’ unique backgrounds and conditions. It is a personalized approach to healthcare that includes environmental and genetic factors that affect health and disease.
“Our goals in the ICTS Precision Health Function are to make genomic resources more accessible to a wide variety of researchers, to facilitate formation of multidisciplinary teams who will apply medical genomics in both bench-to-bedside and bedside-to-bench research, and to educate the genomics research workforce, health care providers, patients, and the public about the use of genomics within the precision health endeavor.” Jenny McKenzie, PhD, Precision Health Program Scientist
What Is Return of Results?
“Anyone who has worked with genetics and recruited patients for genetic studies understands that we, as investigators, have a lot of information in our genomic databases, some of which may be medically relevant and potentially lifesaving to a patient. The ICTS Precision Health function is helping investigators by creating guidelines and resources to actually return important research results back to the patients.” Christina A. Gurnett, MD, PhD, Professor of Neurology, Associate Director of the ICTS
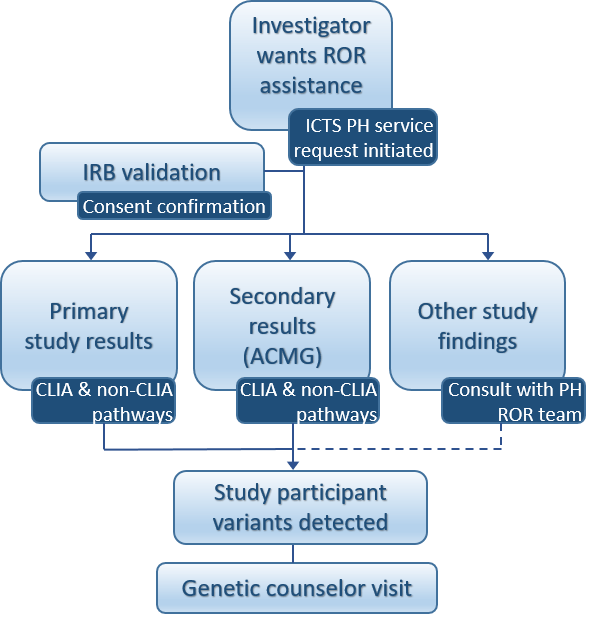
For participants who indicate on their consent form that they would like to be notified of genetic findings that may impact their health, the investigator will return identified findings using the ICTS Precision Health Genomic Return of Results (ROR) Service. The service provides pathways to return primary and secondary genetic findings.
Primary study findings are defined as actionable results found based on the investigator’s original IRB-approved study.
Secondary study findings are a defined set of genes considered medically actionable, even when unrelated to the primary reasons for testing.
Gurnett indicates that what is being built is almost a full-service resource for investigators. “We’ve long known that we may uncover information that is important for participants to know about their health when we do research. For example, we have been returning incidental findings on imaging studies to research participants for many years and would find it unconscionable not to do so.”
Gurnett points out that what hasn’t been as well organized is the process for genetic sequencing studies. “When you do a sequencing study and identify thousands of variants, one of those may actually make someone at risk for a disease, but we haven’t had the resources to return that information back to participants,” said Gurnett. “Part of the challenge is that genetic data accumulates over time and the interpretation of a variant may change. We need to be highly certain that a variant causes a disease and concentrate only on those genes that are clinically actionable, meaning that there is an intervention to prevent or reduce the effects of a disease.”
What Prompted the Need for Return of Results?
Returning results was discussed at WashU as a need, but it wasn’t until the COVID-19 pandemic that ideas on how to best coordinate this effort began to solidify. Community focus groups and the ICTS community advisory board meetings took place during the pandemic when samples from COVID-19-positive patients were being collected in the emergency room.
When investigators began to consider sequencing those patients’ DNA, they pondered if genetic information uncovered through the testing should be returned to patients. Consistently the focus groups and advisory board recommended returning this information and were surprised it was not done automatically. Investigators reported that they would like to return these results but did not have the adequate resources to do so.

“It’s easy to say, ‘I’m going to get someone’s genetic sequence and I want you to tell me if there’s anything they need to know about,’” said Sarah Hartz, MD, PhD, Associate Professor of Psychiatry and ICTS Precision Health Associate Lead. “When you start parsing that out, it quickly becomes complicated. The sequence has a massive amount of data – millions and millions of data points. Someone has to first evaluate the sequencing, and there are multiple types and ways to sequence. Then someone has to take all the data and put it into a pipeline to identify disease causing variants. After that, the disease-causing variants must be confirmed, and then the information needs to be communicated to the participant, including referring them to the right place for next steps. The difficulty has been that there are processes in place in clinical genetics, but if we’re trying to combine that with research sequencing studies, it becomes much more complicated.”
The American College of Medical Genetics and Genomics (ACMG) has compiled a list of 81 genes, for which specific pathogenic variants are known to be causative of disorders with defined phenotypes that are clinically actionable by an accepted intervention. The ACMG recommends that pathogenic variants detected in any of these genes by a method such as whole exome or whole genome sequencing be reported, as they are of medical relevance and could be used in the future to inform clinical treatment. Examples of these genes are the hereditary breast and ovarian cancers, known often as the BRACA1, BRACA2 and PALB2, along with Ehlers-Danlos syndrome and Marfan syndrome.
Returning of these types of results does warrant caution, however. Gurnett stresses that “we want to err on being conservative and only report back true disease-causing variants. We wouldn’t want to cause someone undue alarm by delivering information that isn’t 100% certain to be associated with the disease.”
Outcomes from Using the Return of Results Service
Currently, there are several researchers who have requested to use ROR service, including Tychele Turner, PhD, Assistant Professor of Genetics, who was conducting research in which variants of a new gene responsible for epilepsy were found. Through this service, the medical team was able to provide this information to families as part of the research protocol. As a result, treatment options are being evaluated for the families, potentially moving them into clinical trials or changing their therapy.
Gurnett’s own research involves identifying new disease genes for scoliosis, but it has also identified study participants with a high risk of aortic enlargement due to Marfan syndrome or other related syndromes. The ROR is now reviewing variants that are in the process of being returned to participants.
In addition, many investigators are consulting the ROR, either while in the planning phase of a new sequencing project, or to get advice on how to interpret and return results from ongoing projects.
Genomics Consent

Investigators may spend years collecting data samples, only to find when they were about to use them, that they had not consented participants properly for return of genetic results. The ICTS Precision Health group, led previously by Laura Bierut, MD, developed an IRB-approved, stand-alone consent that permits participants to share their research data and electronic health data in an institutional genomics database. Gurnett shares, “we have individuals that have been consented on this and the data-sharing is possible.”
“The Genomic Return of Results Service is brand new. It was a big effort to put together this process because it is so inter-disciplinary. We needed to establish a team of experts to review the data, and a process for contacting individuals to have them provide a new sample that could be tested in a CLIA lab. Then, to provide access to genetic counseling services, which are available and are part of this service, to explain the result to families. We’re ready to go and our services are now available.” shares Gurnett.
Christina Gurnett, MD, PhD, is the A. Ernest and Jane G. Stein Professor of Developmental Neurology, Director of the Division of Pediatric and Developmental Neurology, and the Chief of Neurology at St Louis Children’s Hospital. She is the Co-Director of the Intellectual and Developmental Disabilities Research Center (IDDRC) and the Associate Director of the Institute of Clinical and Translational Sciences. Gurnett has an active neurogenetics research program focused on gene discovery for neurogenetic disorders and development of deep mutational scanning methods for variant interpretation for rare disease diagnostics. Click for Gurnett’s Lab.