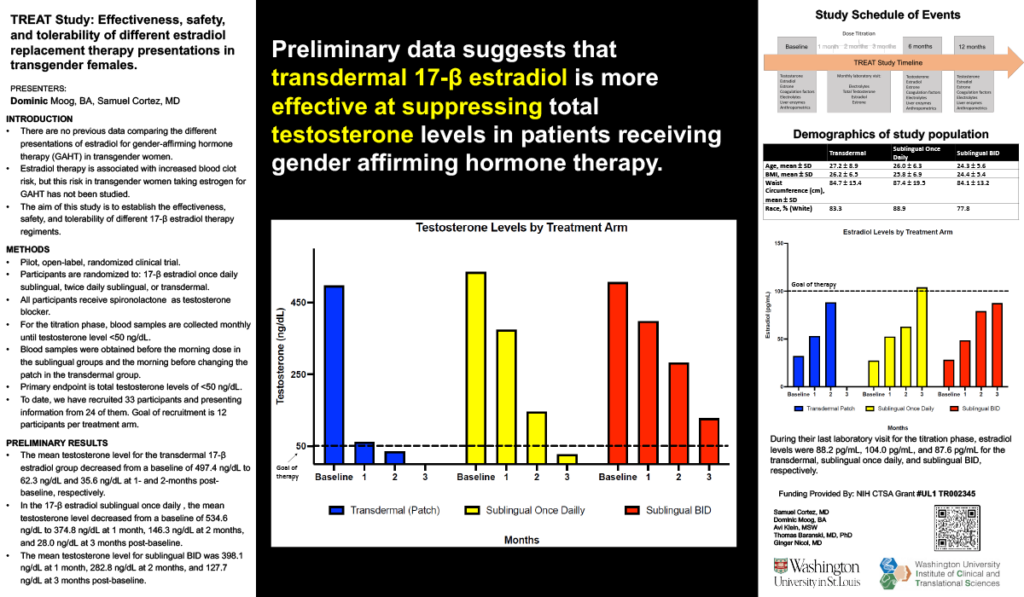
Introduction: A typical regimen for transgender females includes estrogen to provide feminizing effects in conjunction antiandrogens. There is no data to indicate if a single daily dose vs divided daily doses vs continuous subcutaneous dosing of estradiol is more effective at suppressing endogenous testosterone production. The lack of research on gender affirming hormone therapy represents a gap in knowledge that precludes the delivery of safe and appropriate care. The overarching goal of this study is to evaluate the effectiveness and safety of the different estradiol dosing regimens in transgender females. The results will guide the design and implementation of larger clinical trials.
Methods: This is a pilot, open label, randomized clinical trial conducted at the Washington University Transgender Center. Participants are transgender females between the ages of 18 to 45 years. Exclusion criteria includes cigarette smoking, GnRH agonist use, history of blood clot. Participants were randomized to once daily sublingual, twice daily sublingual, or transdermal estradiol. All groups used spironolactone as antiandrogen. Hormone levels were checked monthly, and dose of estradiol and spironolactone were increased until testosterone level was <50 ng/dL.
Results: Study is ongoing. We have recruited 25 participants: 7 in the transdermal group, 9 in the daily sublingual, and 9 in the twice daily sublingual. Baseline characteristics were similar among the three groups. Within the transdermal group, the mean testosterone level was 497.4 ng/dL, 59.1 ng/dL and 37.4 ng/dL at baseline, 4 weeks, and 8 weeks respectively. On the daily sublingual estradiol, the mean testosterone level was 578.3 ng/dL, 390 ng/dL, 146.3 ng/dL at baseline, 4 weeks, 8 weeks, and 12 weeks respectively. On the twice daily sublingual estradiol, the mean testosterone level was 522.8 ng/dL, 398.1 ng/dL, 332.75 ng/dL, and 188 ng/dL at baseline, 4 weeks, 8 weeks, and 12 weeks respectively.
Impact: Preliminary data reflects a better testosterone suppression with the transdermal estradiol route. Further studies are needed to determine if route of administration affects the feminization process.
Organization – Washington University in St. Louis
Cortez S, Moog D, Nicol G, Baranski T