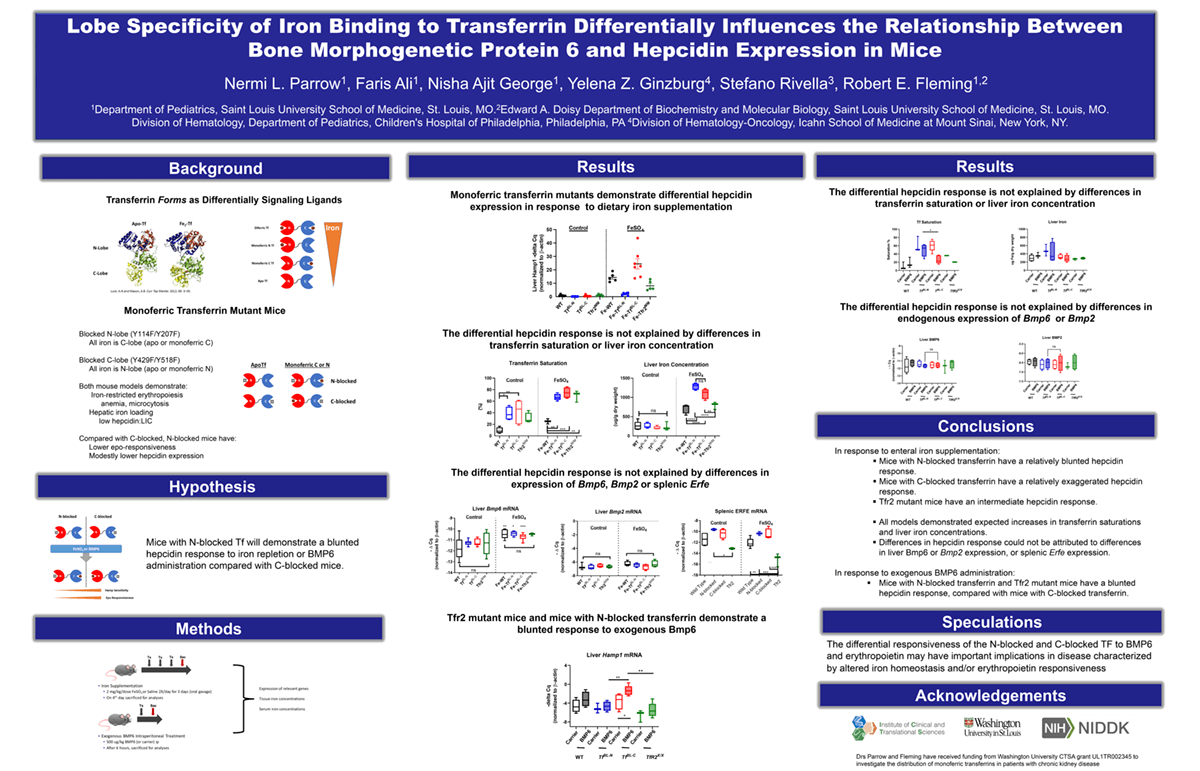
Introduction: The mechanisms by which changes in transferrin (TF) saturation regulate hepcidin are unclear. We reported that transgenic mice expressing mutant TF that block iron binding to either N-lobe (N-bl) or C-lobe (C-bl) have differences in erythropoietin sensitivity and hepcidin regulation. To characterize the differential regulation of the gene encoding hepcidin, Hamp1, in these mice we analyzed the effects of dietary iron or exogenous administration of the iron signaling molecule, bone morphogenetic protein 6 (BMP6).
Methods: Preweanling wild-type (WT), N-bl, and C-bl were gavaged with 4 mg/kg/d FeSO4 (or saline) in 2 divided doses for 3 days and sacrificed on day 4. Other 14-day old mice were administered 500 µg/kg BMP6 (or saline) IP and sacrificed 6 hours later.
Results: Iron-treated mice demonstrated increased serum iron, TF saturation, and liver iron concentration in all 3 groups. The magnitude of increase in Hamp1 mRNA was greater in the C-bl mice (44-fold, p<0.01) compared to N-bl (9-fold) and WT mice (10-fold). The differential effect of iron treatment on Hamp1 was not attributable to effects on liver Bmp6 or Bmp2 expression. It was also not attributable to effects on the expression of splenic Fam132b, the gene encoding the erythroid hepcidin regulator erythroferrone. To further examine the observed increased Hamp1 relative to Bmp6 in the C-blocked mice, we assessed the effect of exogenous BMP6 on Hamp1 expression. The magnitude of the increase in Hamp1 mRNA was likewise greater in C-bl mice compared to N-bl mice and the difference was not attributable to differences in TF saturation, hepatic Bmp6, Bmp2 or splenic Fam132b expression.
Impact: Iron and BMP6 treatment each effected a greater increase in liver Hamp1 expression in TF C-bl compared to N-bl mice. The responsiveness to BMP6 in the regulation of hepcidin is significantly influenced by which TF lobe iron occupies. We speculate that the differential responsiveness of the N-blocked and C-blocked TF to BMP6 and erythropoietin may have important implications in disease characterized by altered iron homeostasis and/or erythropoietin responsiveness.
Organization: Saint Louis University
Parrow NL, Ali F, George NA, Ginzburg YZ, Rivella S, Fleming RE