Humans evolved in a relatively quiet landscape, without natural selection for protection from acoustic trauma. In today’s increasingly loud industrialized world, exposure to noise affects us all, and life is only getting louder. According to findings from the 2011-2012 National Health and Nutrition Examination Survey (NHANES), nearly one in four of US adults aged 20-69 years has some indications of noise-induced hearing loss. The accumulated effects of a lifetime of exposure to moderate levels of sound can accelerate age-related hearing loss, which hits almost everyone.
Statistics such as this highlight the need to prevent or lessen potential hearing loss by blocking the effects of damaging noise levels. By utilizing ICTS resources, Mark A. Rutherford, PhD, assistant professor of otolaryngology at Washington University School of Medicine in St. Louis, is working toward that goal by developing the first drug for prevention of noise-induced hearing loss.
Team Rutherford’s research
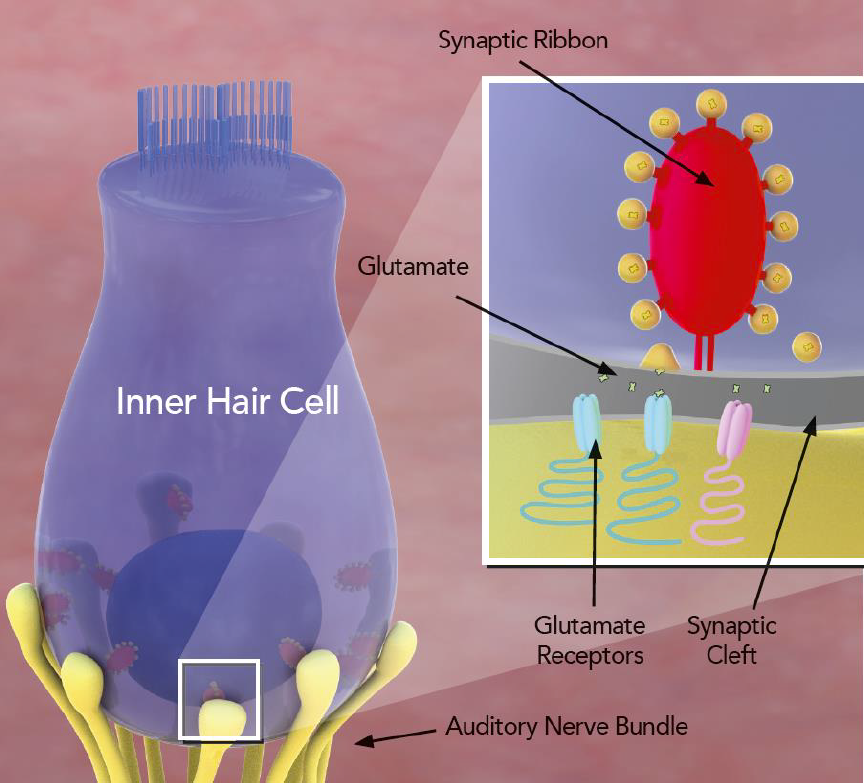
For several years, Rutherford has studied the prevention of hearing loss, specific to the mechanisms of glutamate signaling in the cochlea. Glutamate receptors are necessary for hearing, however, overstimulation of these receptors by loud noise can cause irreversible damage to synapses in the cochlea, thereby causing sensorineural hearing loss. Rutherford’s previous research with a team of investigators including Steven H. Green, PhD, of the University of Iowa, found that glutamate receptors are not all made the same. So, by administering a compound to block some receptors while leaving others unblocked, they found that hearing function could be maintained while preventing damage, as summarized by the SciShow YouTube channel and published in the study “Protection of cochlear synapses from noise-induced excitotoxic trauma by blockade of Ca2+-permeable AMPA receptors” in the February 18, 2020 issue of PNAS.
The drug-based procedure coined “chemical earmuffs” works at the synapse to stop the damage from happening in the first place. Rutherford and colleagues’ research in preliminary studies in mice, funded by the NIH National Institute on Deafness and Other Communication Disorders and the DoD/CDMRP-Hearing Restoration Research Program, gave interest to how potential drug-based therapies could be administered in humans. The team was ready to take the research further.
ICTS Just-In-Time Funding
In 2019, Rutherford applied for the ICTS Just-In-Time Core Usage Funding Program to supplement additional research on his drug discovery journey. He was funded for services from the Washington University Center for Cellular Imaging (WUCCI). Through WUCCI, Rutherford’s team was able to collect data using focused ion beam scanning electron microscopy to study the specialized ‘ribbon’ synapses of the inner ear that connect the cochlea to the auditory nerve. Rutherford noted, “WUCCI’s assistance allowed us to have a clear idea of what the undamaged synapses look like. Now, we were ready to look at therapies to protect those synapses or help them regrow.”
This research was detailed in the study, “Maturation of Heterogeneity in Afferent Synapse Ultrastructure in the Mouse Cochlea” published in the June 17, 2021 issue of Frontiers in Synaptic Neuroscience.
LEAP funding fosters drug discovery research
By spring of 2020, Rutherford and his team initiated a medicinal chemistry strategy to synthesize novel compounds for potential therapies. Rutherford, joined by Steven Green, PhD, at the University of Iowa added Roland Dolle, PhD, chemistry director for the Center of Drug Discovery (CDD) at Washington University School of Medicine to their team and applied to LEAP (Leadership and Entrepreneurial Acceleration Program). The LEAP Gap Fund, supported in part by the ICTS, is open to any person or team with potential or existing Washington University intellectual property and helps to progress early-stage research towards commercialization. Rutherford’s team with their project “Targeting Ca2+-Permeable AMPA Receptors for Inner Ear Therapy” was one of six project teams funded in the spring 2020 LEAP cycle, allowing them to move forward on their first round of medicinal chemistry, followed by compound screening in vitro with the lab of Jim Huettner, PhD, professor of cell biology and physiology, and in vivo with the lab of Alec Salt, PhD, professor of otolaryngology.
Some findings related to the project were published in “Reducing Auditory Nerve Excitability by Acute Antagonism of Ca2+-Permeable AMPA Receptors“, July 5, 2021 in Frontiers in Synaptic Neuroscience.
“LEAP funding allowed us to generate novel compounds to screen, and to show that the commercially available ‘tool’ compounds are metabolically stable and work when given systemically,” commented Rutherford. “LEAP also helped us gain attention from companies interested in collaboration for hearing loss and other indications because Ca2+-permeable AMPA receptors have been implicated in several CNS disorders including stroke, epilepsy, and drug abuse among others.” Studies from other research on AMPA receptors has supported this implication, detailed in “Ca2+-permeable AMPA receptors and their auxiliary subunits in synaptic plasticity and disease”, February 3, 2021 in The Journal of Physiology.
Communicating ICTS resources to fellow investigators
In addition to utilizing ICTS-supported funding, Rutherford takes an active interest in making the most of what the ICTS has to offer, and shares that information with his fellow member investigators. He has served in the past as part of the ICTS Liaison program, charged with sharing information about ICTS resources within his department.

Rutherford has also benefited from ICTS funding programs even when he wasn’t an awardee. “I applied for the Clinical and Translational Research Funding Program (CTRFP) a few years ago,” he recalled. “And although the project wasn’t funded, the CTRFP reviewers provided us with valuable and detailed feedback. You don’t get that kind of feedback with most funding programs. Those critiques improved our approach.”
In the future, Rutherford hopes to try again for the CTRFP and to utilize more Just-In-Time cores for further studies on their drug compounds as the team works toward translating their research into medications that treat noise-induced hearing loss and other pathologies by targeting specific subtypes of glutamate receptors.